Which Condition Is Characterized By Extensive Bone Destruction Followed By Abnormal Bone Repair
Key Messages
-
Osteoporosis affects millions of Americans. Individuals with osteoporosis are at high risk of suffering i or more fractures, which are often physically debilitating and can potentially lead to a downward spiral in concrete and mental wellness.
-
The virtually mutual form of osteoporosis is known every bit "primary osteoporosis." It is the effect of the cumulative impact of bone loss and deterioration of os structure equally people historic period. This bone loss tin be minimized and osteoporosis prevented through adequate diet, concrete activity, and, if necessary, appropriate treatment.
-
There are a wide variety of diseases and certain medications and toxic agents that can cause or contribute to the evolution of osteoporosis. If recognized as a potential threat, this form of the affliction—known as secondary osteoporosis—tin can often be prevented through proper nutrition and physical activeness, along with appropriate therapy if needed.
-
A number of childhood diseases cause rickets, a condition that results from a delay in depositing calcium phosphate mineral in growing bones. This delay leads to skeletal deformities, especially bowed legs. In adults, the equivalent disease is called osteomalacia. Both diseases tin more often than not be prevented by ensuring adequate levels of vitamin D, but they tin can have devastating consequences for affected individuals.
-
Patients with chronic renal affliction are at adventure for developing a complex os disease known as renal osteodystrophy. While dialysis and transplantation have extended the life-expectancy of these patients, information technology may not prevent farther progression of bone affliction.
-
Paget's disease of bone is a progressive, often crippling disorder of bone remodeling that commonly involves the spine, pelvis, legs, or skull (although whatsoever bone tin be affected). If diagnosed early, its impact can be minimized.
-
A large number of genetic and developmental disorders affect the skeleton. Among the more common of these is osteogenesis imperfecta (OI). Patients with this condition have bones that break easily.
-
Some skeletal disorders tend to develop later in life. 1 of the virtually common of these acquired skeletal disorders is a malignancy of the bone. These malignancies can originate in the bone (primary tumors) or, much more commonly, upshot from the seeding of bone past tumors outside of the skeleton (metastatic tumors). Primary bone cancer as well occurs in children. Both types of tumors tin can destroy os.
The body systems that control the growth and maintenance of the skeleton, which are described in Affiliate 2, can be disrupted in different ways that result in a variety of bone diseases and disorders. These include problems that tin can occur at or before birth, such as genetic abnormalities and developmental defects, as well as diseases such as osteoporosis and Paget'southward disease of bone that impairment the skeleton later in life. In addition to weather condition that affect os straight, there are many other disorders that indirectly touch bone by interfering with mineral metabolism. This chapter reviews some of the more common diseases, disorders, and weather condition that both direct and indirectly affect os.
Osteoporosis
As pointed out in Chapter ii, osteoporosis is a disease characterized by low bone mass and deterioration of bone structure that causes bone fragility and increases the risk of fracture. For practical purposes, the World Wellness Organization has defined osteoporosis equally a bone mineral density (BMD) value more than 2.5 standard deviations below the hateful for normal young White women. Osteoporosis is a common disease affecting millions of Americans. As described in Chapters iv and 5, it tin can take devastating consequences. Individuals with osteoporosis are at high risk of suffering ane or more than fractures, injuries that can often exist physically debilitating and potentially lead to a downward spiral in physical and mental health (Figure 3-1). Generalized osteoporosis is the almost common class of the affliction, affecting nigh of the skeleton. Osteoporosis can also occur in localized parts of the skeleton as a result of injury or conditions that reduce muscle forces on the bone, such as limb paralysis. There are a variety of different types of osteoporosis. The most mutual class of osteoporosis is known as "chief osteoporosis"—that is, osteoporosis that is not acquired by some other specific disorder. Bone loss caused past specific diseases or medications (see beneath) is referred to as "secondary osteoporosis." Each of these major categories of osteoporosis is discussed in more particular on the following pages.

Figure three-1
Bone Fracture Areas in Osteoporosis. Source: NOF 2004.
Classical Case
"A classical case of osteoporosis may commencement in a adult female about 55 years of age with a wrist fracture. 10 years later she may nowadays with back pain, with or without minor trauma, and thoracolumbar spine ten-rays may testify a vertebral fracture. She might have i of several risk factors: low torso weight, premature menopause, a family history of fractures, smoking, heavy alcohol consumption, inactivity, calcium or vitamin D deficiency, or corticosteroid use. The back pain may remit and relapse with subsequent vertebral fractures. Approximately ten–xv years later, at the age of 75–80 years, the patient may fall and sustain a hip fracture, resulting in hospitalization, a 20 percentage backlog risk of expiry, considerable functional impairment and possibly a loss of independence if she survives. Although this scenario is instantly recognizable, osteoporosis may present with whatever of a wide range of fractures and at a variety of ages; information technology is also increasingly recognized amid men" (WHO 2003). Recognition that the starting time fracture was a lookout event may have triggered a detailed cess that could potentially take prevented additional fractures. Run across Chapter eight for more information on such assessments.
Master Osteoporosis
Chief osteoporosis is mainly a illness of the elderly, the upshot of the cumulative impact of bone loss and deterioration of os construction that occurs as people historic period (Seeman 2003). This course of osteoporosis is sometimes referred to as age-related osteoporosis. Since postmenopausal women are at greater chance, the term "postmenopausal" osteoporosis is as well used. Younger individuals (including children and immature adults) rarely get primary osteoporosis, although it can occur on occasion. This rare form of the affliction is sometimes referred to as "idiopathic" osteoporosis, since in many cases the exact causes of the disease are not known, or idiopathic. Since the verbal mechanisms by which aging produces bone loss are non all understood (that is, it is not always articulate why some postmenopausal women develop osteoporosis while others do not), age-related osteoporosis is also partially idiopathic. A brief review of "idiopathic" chief osteoporosis and a more detailed review of the more than common status of age-related osteoporosis follows.
Idiopathic Main Osteoporosis
There are several unlike forms of idiopathic osteoporosis that can touch on both children and adolescents, although these weather condition are quite rare (Norman 2003). Juvenile osteoporosis affects previously good for you children betwixt the ages of eight and xiv. Over a period of several years, bone growth is impaired. The condition may be relatively balmy, causing only 1 or 2 complanate basic in the spine (vertebrae), or it may exist severe, affecting nigh the entire spine. The disease almost always goes into remission (spontaneously) around the time of puberty with a resumption of normal bone growth at that time. Patients with balmy or moderate forms of the affliction may be left with a curvature of the spine (kyphosis) and short stature, but those with a more severe form of the affliction may be incapacitated for life.
Primary osteoporosis is quite rare in young adults. In this age-group, the disease is usually caused past another condition or factor, such as anorexia nervosa or glucocorticoid apply (Khosla et al. 1994). When idiopathic forms of primary osteoporosis do occur in young adults, they announced in men equally often equally they practise in women (this is in contrast to historic period-related chief osteoporosis, which occurs more than often in women). The characteristics of the disease can vary broadly and may involve more than than one disorder. Some young adults with idiopathic chief osteoporosis may have a primary defect in the regulation of bone cell function, resulting in depressed os germination, increased bone resorption, or both (see Chapter 2). Others with a mild form of the affliction may simply take failed to reach an adequate amount of skeletal mass during growth. In some patients, the illness runs a mild course, even without treatment, and the clinical manifestations are limited to asymptomatic spinal compression fractures. More than typically, yet, multiple spine fractures occur over a five–10 year period leading to a summit loss of up to 6 inches.
Age-Related Osteoporosis
Age-related osteoporosis is by far the near common course of the disease (Figure 3-2). There are many different causes of the ailment, just the os loss that leads to the disease typically begins relatively early in life, at a time when corrective activity (such as changes in diet and physical activity) could potentially slow down its grade. While it occurs in both sexes, the illness is two to three times more common in women (see Affiliate 4). This is partly due to the fact that women have ii phases of age-related bone loss—a rapid phase that begins at menopause and lasts four–8 years, followed by a slower continuous phase that lasts throughout the rest of life (Riggs et al. 2002). By dissimilarity, men go through merely the slow, continuous phase. Equally a event, women typically lose more bone than practice men. The rapid phase of os loss alone in women results in losses of 5–ten percentage of cortical bone (which makes up the hard outer shell of the skeleton) and 20–30 percent of trabecular os (which fills the ends of the limb bones and the vertebral bodies in the spine, the sites of most osteoporotic fractures). The slow phase of os loss results in losses of twenty–25 percent of cortical and trabecular bone in both men and women, but over a longer period of time (Riggs et al. 2002).

Figure iii-two
Progressive Spinal Deformity in Osteoporosis. Note: Pinch fractures of thoracic vertebrae lead to loss of height and progressive thoracic kyphosis (dowager's hump). Lower ribs eventually residue on ileac crests, and downward pressure on viscera (more...)
Although other factors such as genetics and nutrition contribute, both the rapid stage of bone loss in postmenopausal women and the slow phase of bone loss in crumbling women and men appear to be largely the result of estrogen deficiency. (This is demonstrated by the fact that correction of estrogen deficiency tin prevent these changes.) For women, the rapid phase of bone loss is initiated by a dramatic decline in estrogen production by the ovaries at menopause. The loss of estrogen activeness on estrogen receptors in bone results in large increases in bone resorption (see Chapter 2), combined with reduced bone formation. The stop result is thinning of the cortical outer vanquish of bone and damage to the trabecular os structure (see Figure 2-5, Affiliate ii). There may be some countervailing forces on this process, as the outside bore of the bone can increase with age, thus helping to maintain bone strength (Ahlborg et al. 2003).
By contrast, the slower phase of bone loss is thought to be caused by a combination of factors including age-related impairment of bone formation, decreased calcium and vitamin D intake, decreased concrete activity, and the loss of estrogen's positive furnishings on calcium residual in the intestine and kidney equally well as its effects on bone (Riggs et al. 2002). This leads to further impairment of absorption of calcium by the intestine and reduced ability of the kidney to conserve calcium. If the corporeality of calcium captivated from the diet is insufficient to make up for the obligatory calcium losses in the stool and urine, serum calcium begins to fall. Parathyroid hormone levels will then increase, removing calcium from bone to brand up for the loss, as illustrated in Figure iii-iii. The net result of this process is an increase in bone resorption. It is of import to realize that these mineral losses demand non be cracking to result in osteoporosis. A negative remainder of only 50–100 mg of calcium per day (far less than the 300 mg of calcium in a single glass of milk) over a long period of time is sufficient to produce the affliction.
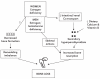
Effigy 3-three
Schematic Representation of Model for Bone Loss in Postmenopausal Women and Aging Men. Source: Riggs et al. 1998.
For aging men, sex steroid deficiency also appears to be a major factor in age-related osteoporosis. Although testosterone is the major sex steroid in men, some of it is converted past the aromatase enzyme into estrogen. In men, however, the deficiency is mainly due to an increase in sexual practice hormone binding globulin, a substance that holds both testosterone and estrogen in a class that is not bachelor for use past the body. Between 30–50 percent of elderly men are scarce in biologically active sex steroids (Khosla et al. 1998). In fact, except for the lack of the early postmenopausal phase, the process of bone loss in older men is similar to that for older women. As with women, the loss of sexual activity steroid action in men has an effect on calcium absorption and conservation, leading to progressive secondary increases in parathyroid hormone levels. As in older women, the resulting imbalance between bone resorption and formation results in slow os loss that continues over life. Since testosterone may stimulate bone formation more than than estrogen does, withal, decreased bone formation plays a relatively greater role in the bone loss experienced past elderly men.
Secondary Osteoporosis
Young adults and fifty-fifty older individuals who get osteoporosis often exercise and so as a byproduct of some other condition or medication use. In fact, there are a broad variety of diseases (Table three-ane) forth with certain medications and toxic agents (Table three-2) that can crusade or contribute to the development of osteoporosis (Stein and Shane 2003). Individuals who get the illness due to these "outside" causes are said to have "secondary" osteoporosis. They typically feel greater levels of os loss than would be expected for a normal individual of the same historic period, gender, and race. Secondary causes of the disease are common in many premenopausal women and men with osteoporosis (Khosla et al. 1994); in fact, by some estimates the majority of men with osteoporosis exhibit secondary causes of the affliction (Orwoll 1998). In addition, up to a 3rd of postmenopausal women with osteoporosis also take other conditions that may contribute to their bone loss (Tannenbaum et al. 2002). This section briefly describes some of the more mutual diseases, disorders, and medications that can cause or contribute to the development of osteoporosis.
Table three-ane
Diseases That Cause or Contribute to Secondary Osteoporosis.
Tabular array 3-ii
Medications Associated With Secondary Osteoporosis.
Diseases and Disorders That Tin Cause Osteoporosis
Several genetic diseases have been linked to secondary osteoporosis. Idiopathic hyper-calciuria and cystic fibrosis are the most common. Patients with cystic fibrosis have markedly decreased bone density and increased fracture rates (Ott and Aitken 1998) due to a variety of factors, including calcium and vitamin D malabsorption, reduced sexual activity steroid production and delayed puberty, and increased inflammatory cytokines (see Chapter 2). Some patients with idiopathic hypercalciuria have a renal defect in the power of the kidney to conserve calcium. This condition may be aggravated if they are brash to lower their dietary calcium intake to forestall kidney stones. Several studies have documented low bone density in these individuals, and they may respond to drugs that subtract calcium excretion in the urine. Other genetic disorders (listed in Table 3-i), although rare, should be considered in patients with osteoporosis after more than common causes have been excluded.
Estrogen or testosterone deficiency during adolescence (due to Turner's, Kallman's, or Klinefelter's syndrome, anorexia nervosa, athletic amenorrhea, cancer, or any chronic illness that interferes with the onset of puberty) leads to depression peak bone mass (Riggs et al. 2002). Estrogen deficiency that develops subsequently peak bone mass is achieved but before normal menopause (due to premature ovarian failure for example) is associated with rapid os loss. Low sex activity steroid levels may also be responsible for reduced bone density in patients with androgen insensitivity or acromegaly. By contrast, excess thyroid hormone (thyrotoxicosis), whether spontaneous or caused by overtreatment with thyroid hormone, may be associated with substantial bone loss (Ross 1994); while bone turnover is increased in these patients, os resorption is increased more than than bone germination. Likewise, backlog production of glucocorticoids acquired past tumors of the pituitary or adrenal glands (Cushing's syndrome) tin can lead to speedily progressive and severe osteoporosis, as can treatment with glucocorticoids (see below). The relationship between diabetes and osteoporosis is more controversial (Stein and Shane 2003). For example, hip fractures are increased in some studies of diabetic patients, simply not in others. In general, patients with type 1 (insulin-dependent) diabetes, peculiarly those with poor control of their blood sugar (Heap et al. 2004), are at greater chance of osteoporosis than are those with type 2 (non-insulin dependent) diabetes (Piepkorn et al. 1997).
Primary hyperparathyroidism is a relatively mutual condition in older individuals, specially postmenopausal women, that is caused by excessive secretion of parathyroid hormone. Virtually oft, the cause is a benign tumor (adenoma) in one or more parathyroid glands; very rarely (less than 0.v percent of the time) the cause is parathyroid cancer (Wynne et al. 1992). Since most patients at present come up to clinical attending when they are unexpectedly establish on routine examination to have an abnormally high calcium level in the claret (Wermers et al. 1997), the clinical presentation has changed over the by thirty years from an uncommon but highly symptomatic disorder involving renal stones and bone affliction (osteitis fibrosa cystica) to a common only relatively asymptomatic condition (Silverberg and Bilezikian 2001). Typically, cortical bone (for example, in the distal forearm) is affected to a greater extent than trabecular bone (for example, in the spine) in primary hyperparathyroidism (Silverberg et al. 1989). It is presumed that the reduction in bone mass is associated with the increased adventure of fracture seen in these patients (Khosla and Melton 2002).
Diseases that reduce intestinal assimilation of calcium and phosphorus, or impair the availability of vitamin D, can also cause os illness. Moderate malabsorption results in osteoporosis, merely severe malabsorption may cause osteomalacia (see below). Celiac disease, due to inflammation of the small-scale intestine by ingestion of gluten, is an important and commonly disregarded cause of secondary osteoporosis (Bianchi and Bianchi 2002). Likewise, osteoporosis and fractures take been establish in patients following surgery to remove function of the stomach (gastrectomy), specially in women. Os loss is seen afterward gastric bypass surgery even in morbidly obese women who exercise not have depression bone mass initially (Coates et al. 2004). Increased osteoporosis and fractures are likewise seen in patients with Crohn's disease and ulcerative colitis (Bernstein et al. 2000). Glucocorticoids, ordinarily used to treat both disorders, probably contribute to the os loss. Similarly, diseases that impair liver function (main biliary cirrhosis, chronic active hepatitis, cirrhosis due to hepatitis B and C, and alcoholic cirrhosis) may issue in disturbances in vitamin D metabolism and may likewise cause os loss past other mechanisms. Primary biliary cirrhosis is associated with particularly astringent osteoporosis. Fractures are more frequent in patients with alcoholic cirrhosis than any other types of liver disease, although this may be related to the increased risk of falling among heavy drinkers (Crawford et al. 2003). Human immunodeficiency virus (HIV) infected patients also have a higher prevalence of osteopenia or osteoporosis (Brown et al. 2004). This may involve multiple endocrine, nutritional, and metabolic factors and may also be afflicted past the antiviral therapy that HIV patients receive (Thomas and Doherty 2003).
Autoimmune and allergic disorders are associated with bone loss and increased fracture risk. This is due not simply to the effect of immobilization and the damage to bone by the products of inflammation from the disorders themselves, just likewise from the glucocorticoids that are used to treat these atmospheric condition (Lien et al. 2003, Orstavik et al. 2004). Rheumatic diseases similar lupus and rheumatoid arthritis have both been associated with lower bone mass and an increased hazard of fractures. A study institute that 12 percent of women with systemic lupus erythematosus reported at to the lowest degree i fracture since the onset of affliction, a 4.7-fold college risk of fracture than for the typical woman. Fractures in these women were found to exist associated with the following: older age at diagnosis, longer disease duration, longer duration of steroid use, and post-menopausal condition (Ramsey-Goldman et al. 1999, Haugeberg et al. 2003).
Many neurologic disorders are associated with dumb os health and an increased risk of fracture (Whooley, Kip et al. 1999; Lloyd, Spector et al. 2000). This may be due in function to the effects of these disorders on mobility and balance or to the effects of drugs used in treating these disorders on bone and mineral metabolism. Unfortunately, withal, wellness care providers often fail to assess the bone health of patients who have these disorders or to provide advisable preventive and therapeutic measures. For case, patients with stroke, spinal cord injury, or neurologic disorders prove rapid bone loss in the affected areas (Dauty, Perrouin Verbe et al. 2000; Poole, Reeve et al. 2002; Tuzun, Altintas et al. 2003). There are many disabling conditions that tin can pb to bone loss, and thus information technology is important to pay attention to bone wellness in patients with developmental disabilities, such as cerebral palsy, as well as diseases affecting nerve and musculus, such as poliomyelitis and multiple sclerosis. Children and adolescents with these disorders are unlikely to attain optimal meridian os mass, due both to an increase in bone resorption and a decrease in bone formation. In some cases very rapid bone loss tin can produce a large enough increase in blood calcium levels to produce symptoms (Carey and Raisz 1985; Go 2001). Fractures are common in these individuals not only because of os loss, but besides because of muscular weakness and neurologic harm that increases the likelihood of falls. Bone loss can be slowed—but not completely prevented—by antiresorptive therapy (Sato, Asoh et al. 2000). Epilepsy is another neurologic disorder that increases the risk of os disease, primarily because of the adverse furnishings of anti-epileptic drugs. Many of the drugs used in epilepsy can impair vitamin D metabolism, probably by interim on the liver enzyme which converts vitamin D to 25 hydroxy vitamin D (Farhat, Yamout et al. 2000, Sheth 2002). In addition, there may exist a straight effect of these agents on bone cells. Due to the negative bone-health effects of drugs, near epilepsy patients are at risk of developing osteoporosis. In those who have low vitamin D intakes, intestinal malabsorption, or depression sun exposure, the boosted effect of anti-epileptic drugs can pb to osteomalacia. Supplemental vitamin D may be effective in slowing bone loss, although patients who develop osteoporosis may require additional therapy such every bit bisphosphonates.
Psychiatric disorders can also accept a negative impact on bone health. While anorexia nervosa is the psychiatric disorder that is nigh regularly associated with osteoporosis, major depression, a much more common disorder, is also associated with low os mass and an increased run a risk of fracture (Coelho, Silva et al. 1999; Cizza, Ravn et al. 2001; Robbins, Hirsch et al. 2001). Many studies show lower BMD in depressed patients (Michelson et al. 1996). In improver, one large report found an increased incidence of falls and fractures among depressed women, even though there was no departure between their BMD and that of non-depressed women included in the study (Whooley, Kip et al. 1999). Higher scores for depressive symptoms accept likewise been reported in women with osteoporosis. Still what these studies practise not brand clear is whether major depression causes depression BMD and increased fracture risk, or whether the depression is a consequence of the diminished quality of life and disability that occurs in many osteoporotic patients. One gene that may crusade os loss in severely depressed individuals is increased product of cortisol, the adrenal stress hormone. Whatsoever the cause of depression BMD and increased fracture risk, measurement of BMD is appropriate in both men and women with major low. While the response of individuals with major low to calcium, vitamin D, or antiresorptive therapy has not been specifically documented, it would seem reasonable to provide these preventive measures to patients at high run a risk.
Finally, several diseases that are associated with osteoporosis are not hands categorized. Aseptic necrosis (also called osteonecrosis or avascular necrosis) is a well-known skeletal disorder that may exist a complication of injury, treatment with glucocorticoids, or alcohol abuse (Pavelka 2000). This condition commonly affects the ends of the femur and the humerus. The precise cause is unknown, just at least 2 theories have been suggested. One is that claret supply to the bone is blocked by collapsing os. The other is that microscopic fatty particles block claret period and outcome in os cell death. Chronic obstructive pulmonary affliction (emphysema and chronic bronchitis) is besides at present recognized equally being associated with osteoporosis and fractures even in the absence of glucocorticoid therapy. Immobilization is clearly associated with rapid os loss; patients with spinal string lesions are at particularly high risk for fragility fractures (Kiratli 2001). However, even pocket-size reductions in physical activity tin lead to bone loss (come across Chapter 6). Hematological disorders, peculiarly malignancies, are commonly associated with osteoporosis and fractures as well. These are discussed in more detail later in the chapter.
Medications and Therapies That Can Cause Osteoporosis
Osteoporosis can as well be a side outcome of particular medical therapies (Table three-2).
Glucocorticoid-Induced Osteoporosis (GIO). GIO is by far the nearly common form of osteoporosis produced past drug handling. While it has been known for many years that excessive production of the adrenal hormone cortisol can cause thinning of the bone and fractures, this condition, a form of Cushing's syndrome, remains uncommon. With the increased use of prednisone and other drugs that human action like cortisol for the treatment of many inflammatory and autoimmune diseases, this form of bone loss has become a major clinical business. The concern is greatest for those diseases in which the inflammation itself and/ or the immobilization acquired by the illness likewise caused increased bone loss and fracture risk. Glucocorticoids, which are used to treat a wide diversity of inflammatory conditions (east.chiliad., rheumatoid arthritis, asthma, emphysema, chronic lung disease), tin can cause profound reductions in bone formation and may, to a bottom extent, increase bone resorption (Saag 2002), leading to loss of trabecular bone at the spine and hip, especially in postmenopausal women and older men. The almost rapid bone loss occurs early in the grade of treatment, and fifty-fifty small doses (equivalent to 2.5–7.five mg prednisone per twenty-four hours) are associated with an increment in fractures (van Staa et al. 2002). As shown in Figure 3-4, the risk of fractures increases apace in patients treated with glucocortocoids, even before much os has been lost. This rapid increment in fracture risk is attributed to damage to the bone cells, which results in less salubrious bone tissue. To avoid this trouble, health intendance providers are urged to use the lowest possible dose of glucocorticoids for equally short a time equally possible. For some diseases, providers should also consider giving glucocorticoids locally (e.g., asthma patients can inhale them), which results in much less harm to the bone.

Figure 3-4
Rapid Increase in Vertebral Fracture Rates in Patients Treated With Glucocorticoids. Notation: Before glucocorticoid treatment was started, the rate of fractures was less than 0.two% per year. On the college doses (more than than 7.5 mg of prednisone or equivalent (more...)
Other Medications That Can Cause Osteoporosis. Cyclosporine A and tacrolimus are widely used in conjunction with glucocorticoids to forbid rejection after organ transplantation, and high doses of these drugs are associated with a particularly astringent grade of osteoporosis (Cohen and Shane 2003). Os disease has also been reported with several oftentimes prescribed anticonvulsants, including diphenylhydantoin, phenobarbital, sodium valproate, and carbamazepine (Stein and Shane 2003). Patients who are most at take a chance of developing this blazon of bone disease include those on long-term therapy, high medication doses, multiple anticonvulsants, and/or simultaneous therapy with medications that raise liver enzyme levels. Low vitamin D intake, restricted sun exposure, and the presence of other chronic illnesses increment the chance, specially amongst elderly and institutionalized individuals. In contrast, high intakes of vitamin A (retinal) may increase fracture risk (Michaelsson et al 2003). Methotrexate, a folate antagonist used to treat malignancies and (in lower doses) inflammatory diseases such as rheumatoid arthritis, may also cause os loss, although research findings are not consistent. In addition, gonadotropin-releasing hormone (GnRH) agonists, which are used to treat endometriosis in women and prostate cancer in men, reduce both estrogen and testosterone levels, which may crusade significant bone loss and fragility fractures (Smith 2003).
Rickets and Osteomalacia
Rickets (which affects children) and osteomalacia (which affects adults) are relatively uncommon diseases in the United States, since they can by and large be prevented by ensuring adequate levels of vitamin D. These diseases can have devastating consequences to those who get them (Chesney 2001, Pettifor 2002 and 2003).
A number of childhood diseases cause rickets, a condition that results from a delay in depositing calcium phosphate mineral in growing bones, thus leading to skeletal deformities, especially bowed legs. In adults, the equivalent disease is called osteomalacia. Since longitudinal growth has stopped in adults, scarce bone mineralization does non crusade skeletal deformity simply can lead to fractures, particularly of weight-bearing bones such as the pelvis, hip, and feet. Even when in that location is no fracture, many patients with rickets and osteomalacia suffer from bone pain and can experience severe muscle weakness.
Rickets and osteomalacia are typically caused by any of a variety of environmental abnormalities. While rare, the disorder tin can also be inherited (Drezner 2003) as a result of mutations in the factor producing the enzyme that converts 25-hydroxy vitamin D to the active form, one,25-dihydroxy vitamin D, or in the factor responsible for the vitamin D receptor. Osteomalacia tin can also be caused by disorders that cause marked loss of phosphorus from the torso. This can agree as a congenital disorder or can be acquired in patients who have tumors that produce a protein that affects phosphorus transport in the kidney.
Since vitamin D is formed in the skin past sunlight, the most common cause is reduced sunday exposure. This is particularly important in northern latitudes where the winter sun does not have the power to form vitamin D in the pare. Thus the disease is often seen in individuals living at northern latitudes, specially immigrants who accept pigmented skin that decreases the formation of vitamin D or who habitually comprehend themselves. This problem tin can also occur in children who are confined indoors and in individuals who are business firm-bound (e.one thousand., due to chronic ill wellness or frailty). Patients with diseases of the gastrointestinal tract, such as gastrectomy, malabsorption syndromes, and small bowel resection, are likewise at college take chances, since these weather reduce vitamin D absorption from the diet.
There is also a second course of rickets and osteomalacia that is caused by phosphate deficiency. This status can be inherited (this is known as X-linked hypophosphatemic rickets), merely it is more than commonly the result of other factors. Individuals with diseases affecting the kidney's power to retain phosphate rapidly are at risk of this condition, every bit are those with diseases of the renal tubule that affect the site of phosphate reabsorption. While nigh foods are rich in phosphate, phosphate deficiency may also effect from consumption of very large amounts of antacids containing aluminum hydroxide, which prevents the absorption of dietary phosphate. Finally, rickets due to phosphate deficiency may occur in individuals with caused or inherited defects in acid secretion by the kidney tubule and those who take certain drugs (Table 3-iii) that interfere with phosphate absorption or the os mineralization procedure.
Tabular array three-three
Causes of Drug-Induced Rickets/Osteomalacia.
There are likewise patients who develop tumors that secrete a factor that causes loss of phosphate from the body. This condition is chosen tumor-induced or oncogenic osteomalacia.
Renal Osteodystrophy
Patients with chronic renal disease are non but at run a risk of developing rickets and osteomalacia (Elder 2002), just they are besides at risk of a complex os illness known every bit renal osteodystrophy (Cunningham et al. 2004). This condition is characterized past a stimulation of bone metabolism caused by an increase in parathyroid hormone and by a filibuster in bone mineralization that is caused by decreased kidney product of i,25-dihydroxyvitamin D. In addition, some patients show a failure of bone formation, chosen adynamic bone disease. Equally a result of this complexity, bone biopsies are often needed to brand a right diagnosis (Martin et al. 2004). By the time the patient progresses to stop-phase renal failure, clinical manifestations of the illness announced, including bone cysts that event from stimulation of osteoclasts by the excess parathyroid hormone. While dialysis can significantly extend the life-expectancy of patients with chronic renal failure, it does cipher to prevent further progression of the osteodystrophy. In fact, the managing of the patient through dialysis may pb to further bone abnormalities that go superimposed on the underlying osteodystrophy, thus increasing the hazard of fractures (Alem et al. 2000). While a renal transplant (offered to a growing number of patients on dialysis) may reverse many features of renal osteodystrophy, the use of antirejection medication in transplant patients may cause bone loss and fractures.
Paget'south Disease of Bone
Paget's affliction of bone (Siris and Roodman 2003) is a progressive, often crippling disorder of bone remodeling (meet Chapter 2) that normally involves the spine, pelvis, legs, or skull (although whatever bone can exist affected). If diagnosed early, its impact tin be minimized. Individuals with this condition feel an increment in os loss at the affected site due to excess numbers of overactive osteoclasts. While os formation increases to compensate for the loss, the rapid production of new bone leads to a disorganized construction. The resulting bone is expanded in size and associated with increased formation of blood vessels and connective tissue in the bone marrow. Such bone becomes more susceptible to deformity or fracture (Figure 3-5). Depending on the location, the status may produce no clinical signs or symptoms, or it may be associated with bone pain, deformity, fracture, or osteoarthritis of the joints next to the aberrant bone. Paget'southward disease of bone can too cause a variety of neurological complications as a result of pinch of nerve tissue by pagetic bone. In very rare cases (probably less than 1 percentage of the fourth dimension) the disease is complicated by the development of an osteosarcoma.

Figure 3-5
Paget's Affliction of Bone. Note: On left, radiograph of humerus showing pagetic change in the distal half, with cortical thickening, expansion, and mixed areas of lucency and sclerosis, contrasted with normal bone in the proximal one-half. On correct, (more...)
Although Paget's disease is the second well-nigh common bone affliction after osteoporosis (see Chapter 4), many questions remain regarding its pathogenesis. There is a potent familial predisposition for Paget's disease, merely no single genetic abnormality has been identified that can explain all cases. Paget'due south disease can be transmitted (or inherited) across generations in an affected family unit; fifteen–40 percent of patients take a relative with the disorder (Morales-Piga et al. 1995). Studies in the U.s. (Siris et al. 1991) suggest that a close relative of a pagetic patient is seven times more than probable to develop Paget'southward disease than is someone who does non accept an affected relative. Notwithstanding, environmental factors are likely play a function in the majority of cases. For instance, some studies take suggested that Paget'southward illness may result from a "boring virus" infection with measles (Friedrichs et al. 2002).
Paget's Disease of Bone
Paget's affliction may present in many different means since it can affect bones throughout the trunk. A typical case might exist a human in his 60s who complains to his doctor of pain in the hip. The doc might tell him he has arthritis and propose that he take ibuprofen or acetaminophen (Tylenol). So, several years afterwards, a routine screening may show a high alkaline phosphatase level. This examination would so prompt use of a bone scan and radiographs, which would finally prove Paget's affliction of his femur and pelvic bone. Unfortunately, by this time the homo probable has developed some bowing of the leg and suffered damage to the joints, neither of which can exist reversed by treatment. Still, treatment with a bisphosphonate can stop the progression of the affliction. As a event, the man lives the balance of his life with some pain and he walks with a limp. Not surprisingly, the homo, his family, and his dr. all wish that the diagnosis had been made earlier. Since Paget'due south disease runs in families, they decide to test the man's relatives. These tests evidence that the man's younger brother has a similar problem. He is treated immediately and no deformities ever develop.
Developmental Skeletal Disorders
A large number of genetic and developmental disorders affect the skeleton. Among the more common and more of import of these is a group of inherited disorders referred to as osteogenesis imperfecta or OI (Whyte 2003, Rauch and Glorieux 2004). Patients with this condition have bones that break easily (therefore, the condition is also known as brittle bone illness). There are a number of forms of OI (see Table 3-four) that outcome from dissimilar types of genetic defects or mutations. These defects interfere with the body's production of type I collagen, the underlying protein structure of bone. Every bit illustrated in Table iii-iv, well-nigh, but not all, forms of OI are inherited. The disease manifests through a diverseness of clinical signs and symptoms, ranging from severe manifestations that are incompatible with life (that is, causing a stillbirth) to a relatively asymptomatic affliction. Withal, about OI patients accept low bone mass (osteopenia) and every bit a result suffer from recurrent fractures and resulting skeletal deformities. There are four chief types of OI, which vary according to the severity and duration of the symptoms. The most common form (Type I) is also the mildest version; and patients may have relatively few fractures. The second mildest course of the disease (which is called Type Four, because it was the fourth type of OI to be discovered) results in mild to moderate os deformity, and sometimes in dental problems and hearing loss. These patients as well sometimes have a blueish, purple, or greyness discoloration in the whites of their eyes, a status known as blueish sclera. A more severe course of the illness (Type Three) results in relatively frequent fractures, and often in brusk stature, hearing loss, and dental problems. Finally, patients with the most severe form of the disease (Type 2) typically endure numerous fractures and severe bone deformity, generally leading to early death.
Table 3-4
Clinical Heterogeneity and Biochemical Defects in Osteogenesis Imperfecta (OI).
OI is non the only group of developmental skeletal disorders. An even larger group of rare diseases (sclerosing bone disorders) causes an increase in bone mass (Whyte 2003). I of these, osteopetrosis (marble os illness), is more or less the opposite of osteoporosis. Instead of overactive osteoclasts, osteopetrosis results from a variety of genetic defects that impair the power of osteoclasts to resorb bone. This interferes with the normal development of the skeleton and leads to excessive bone accumulation. Although such bone is very dense, information technology is also brittle and thus fractures often result. In add-on, past compressing various nerves, the excess os in patients with osteopetrosis may cause neurological symptoms, such as deafness or incomprehension. These patients may also suffer anemia, as blood-forming cells in the os marrow are "crowded out" past the backlog os. Like symptoms can consequence from overactivity of these bone cells, as in fibrous dysplasia where os-forming cells produce also much connective tissue.
Malignancy and the Skeleton
Some other skeletal disorders are non inherited but rather develop only later in life. One of the most mutual of these caused skeletal disorders is a tumor of the bone. Bone tumors can originate in the bone (these are known equally principal tumors) or, much more commonly, consequence from the seeding of os by tumors outside of the skeleton (these are known as metastatic tumors, since they have spread from elsewhere). Both types of tumors tin can destroy bone, although some metastatic tumors can actually increase os formation. Primary bone tumors can exist either beneficial (noncancerous) or malignant (cancerous). The well-nigh common beneficial bone tumor is osteochondroma, while the near mutual malignant ones are osteosarcoma and Ewing's sarcoma. Metastatic tumors are often the result of breast or prostate cancer that has spread to the bone (Coleman 2001). These may destroy bone (osteolytic lesion) or cause new bone formation (osteoblastic lesion). Chest cancer metastases are usually osteolytic, while most prostate cancer metastases are osteoblastic, though they yet destroy bone structure (Berruti et al. 2001). Many tumor cells produce parathyroid hormone related peptide, which increases bone resorption (Bryden et al. 2002). This process of tumor-induced bone resorption leads to the release of growth factors stored in bone, which in turn increases tumor growth yet further.
Osteogenesis Imperfecta (OI)
There is an enormous range of severity in OI cases, from children who are stillborn due to multiple fractures in the womb and the inability to exhale, to children who suffer a few fractures, to mild cases where fractures do not occur until later in life, much like in patients with osteoporosis.
1 scenario that causes tremendous hardship for the patient and the family unit is the occurrence of multiple fractures in the offset few years of life in a kid without the telltale sign of OI—a blue color in the "whites" of their eyes. Often the parents of these children are accused of child abuse. While it may exist possible to brand a diagnosis by analyzing the child'south tissues, this expensive, difficult-to-perform test is not widely available. Typically, the frequency of fractures decreases over fourth dimension and may even stop entirely at puberty. Equally an adult, an OI patient may be left with considerable deformity and brusk stature, just he or she can generally office well with the right environment and back up. When females with OI reach menopause they sometimes first to fracture once again. Since treatment is now available, it is of import to identify OI patients at all stages of life, and to alert family unit members of the possibility that they may also be affected. Pediatricians, orthopedists, emergency room physicians, and others who run across children with fractures need to consider OI as a possible cause, specially in cases involving multiple fractures or a family history of fractures. These points are covered in detail in a recent book (Chiasson et al. 2004).
Bone devastation besides occurs in the vast majority (over 80 percent) of patients with some other type of cancer, multiple myeloma, which is a malignancy of the plasma cells that produce antibodies (Berenson 2002). The myeloma cells secrete cytokines (see Affiliate 2), substances that may stimulate osteoclasts and inhibit osteoblasts (Roodman 2001, Tian et al. 2003). The bone destruction tin cause severe bone hurting, pathologic fractures, spinal cord compression, and life-threatening increases in blood calcium levels (Callander and Roodman 2001). A beneficial form of overproduction of antibodies, called monoclonal gammopathy, may also be associated with increased fracture gamble (Melton et al. 2004).
Bone-resorbing cytokines are too produced in astute and chronic leukemia, Burkitt'south lymphoma, and non-Hodgkins'south lymphoma; patients with these chronic lymphopro-liferative disorders oft have associated osteoporosis. Both osteoporosis and osteosclerois (thickening of trabecular bone) accept been reported in association with systemic mastocytosis, a condition of abnormal mast prison cell proliferation (Schneider and Shane 2001). In add-on, there are other infiltrative processes that bear on os, including infections and marrow fibrosis (myelofibrosis).
Oral Wellness and Bone Disease
Oral bone, like the rest of the skeleton, comprises both trabecular and cortical bone and undergoes formation and resorption throughout the life span. When oral bone loss exceeds gain, it can cause a loss of tooth-anchoring back up or it can diminish the remaining ridge in those areas where partial or consummate tooth loss has occurred.
The prevalence of oral bone loss is pregnant among adult populations worldwide, and it increases with age for both sexes. Oral bone loss and attendant tooth loss are associated with estrogen deficiency and osteoporosis. As a consequence, osteoporosis or osteopenia in postmenopausal women may have an bear on on the demand for, and the outcomes from, a variety of periodontal and prosthetic procedures, including guided tissue regeneration and molar implantation. Furthermore, it is possible that oral examination and radiographic findings may exist useful signs of extra-oral bone loss (Jeffcoat et al. 2000, Geurs et al. 2000).
Key Questions for Futurity Research
The major diseases of bone have been broadly characterized, only many questions remain unanswered, as outlined below:
-
Within the spectrum of clinical disorders that represent "primary osteoporosis," are there differences in the mechanisms that lead to bone loss and bone fragility? What implications do these differences have for diagnosis and handling?
-
How do the environmental and genetic determinants of bone mass and strength collaborate in individuals with certain diseases? For example, are there genetic differences in the response to estrogen or calcium deficiency that affect their relative importance in the pathogenesis of osteoporosis?
-
Are the brute studies on the role of cytokines relevant to human disease?
-
What are the implications of research on the pathogenetic mechanisms for diseases other than osteoporosis? For example, farther enquiry on Paget's affliction could uncover more than nigh the ways in which excessive osteoclastic bone resorption can occur.
-
What is the role of phosphate in os mineralization?
-
How is bone afflicted in patients who have cancer? What implications do these changes accept with respect to both the spread of the cancer and to other skeletal disorders?
References
-
Ahlborg HG, Johnell O, Turner CH, Rannevik Chiliad, Karlsson MK. Bone loss and os size afterward menopause. N Engl J Med. 2003 Jul 24;349(4):327–34. [PubMed: 12878739]
-
Alem MA, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C. Increased risk of hip fracture amid patients with stop-stage renal disease. Kidney Int. 2000;58(1):396–9. [PubMed: 10886587]
-
Berenson JR. Advances in the biology and handling of myeloma bone illness. Semin Oncol. 2002 December;29(6 Suppl 17):11–six. [PubMed: 12520479]
-
Bernstein CN, Blanchard JF, Leslie W, Wajda A, Yu BN. The incidence of fracture amongst patients with inflammatory bowel disease. A population-based accomplice written report. Ann Intern Med. 2000 Nov 21;133(10):795–9. [PubMed: 11085842]
-
Berruti A, Dogliotti Fifty, Tucci K, Tarabuzzi R, Fontana D, Angeli A. Metabolic os disease induced by prostate cancer: Rationale for the utilise of bisphosphonates. J Urol. 2001 December;166(6):2023–31. [PubMed: 11696699]
-
Bianchi ML, Bardella MT. Bone and celiac disease. Calcif Tissue Int. 2002 Dec;71(6):465–71. [PubMed: 12232681]
-
Bilezikian JP. Master hyperparathyroidism. In: Favus MJ, editor. Primer on the metabolic os diseases and disorders of mineral metabolism. v. Washington (DC): American Society for Bone and Mineral Research; 2003. pp. 230–5.
-
Brown TT, Ruppe MD, Kassner R, Kumar P, Kehoe T, Dobs Every bit, Timpone J. Reduced bone mineral density in homo immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia. J Clin Endocrinol Metab. 2004 Mar;89(3):1200–vi. [PubMed: 15001610]
-
Bryden AA, Hoyland JA, Freemont AJ, Clarke NW, George NJ. Parathyroid hormone related peptide and receptor expression in paired primary prostate cancer and bone metastases. Br J Cancer. 2002 Feb 1;86(iii):322–5. [PMC free article: PMC2375222] [PubMed: 11875691]
-
Byers PH. Disorders of collagen biosynthesis and structure. In: Scriver CR, Beaudet AL, Sly WA, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: The McGraw Hill Companies; 2001. pp. 5241–85.
-
Callander NS, Roodman GD. Myeloma os disease. Semin Hematol. 2001 Jul;38(three):276–85. [PubMed: 11486316]
-
Carey DE, Raisz LG. Calcitonin therapy in prolonged immobilization hypercalcemia. Arch Phys Med Rehabil. 1985 Sep;66(9):640–4. [PubMed: 4038033]
-
Chesney RW. Vitamin D deficiency and rickets. Rev Endocr Metab Disord. 2001 April;2(2):145–51. [PubMed: 11705320]
-
Chiasson RM, Munns C, Zeitlin 50. Interdisciplinary treatment arroyo for children with osteogenesis imperfecta. Montreal, Canada: Shriner's Hospitals for Children; 2004.
-
Cizza Thou, Ravn P, Chrousos GP, Gilded Prisoner of war. Low: A major, unrecognized risk factor for osteoporosis. Trends Endocrinol Metab. 2001 Jul;12(5):198–203. [PubMed: 11397644]
-
Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increment in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004 Mar;89(three):1061–5. [PubMed: 15001587]
-
Coelho R, Silva C, Maia A, Prata J, Barros H. Bone mineral density and depression: A customs report in women. J Psychosom Res. 1999 Jan;46(1):29–35. [PubMed: 10088979]
-
Cohen A, Shane Due east. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int. 2003 Aug;14(eight):617–xxx. Epub 2003 Aug 08. [PubMed: 12908095]
-
Coleman RE. Metastatic os disease: Clinical Features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001 Jun;27(three):165–76. [PubMed: 11417967]
-
Crawford BA, Kam C, Donaghy AJ, McCaughan GW. The heterogeneity of bone illness in cirrhosis: A multivariate assay. Osteoporos Int. 2003 December;xiv(12):987–94. [PubMed: 14504696]
-
Cunningham J, Sprague SM, Cannata-Andia J, Coco Chiliad, Cohen-Solal M, Fitzpatrick 50, Goltzmann D, Lafage-Proust MH, Leonard M, Ott South, Rodriguez M, et al. Osteoporosis in chronic kidney illness. Am J Kidney Dis. 2004 Mar;43(iii):566–71. [PubMed: 14981616]
-
Dauty One thousand, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal string-injured patients. Bone. 2000 Aug;27(2):305–ix. [PubMed: 10913927]
-
Drezner MK. Hypophosphatemic rickets. Endocr Dev. 2003;six:126–55. [PubMed: 12964430]
-
Elder G. Pathophysiology and recent advances in the management of renal osteodystrophy. J Os Miner Res. 2002 December;17(12):2094–105. [PubMed: 12469904]
-
Farhat Grand, Yamout B, Mikati MA, Demirjian Southward, Sawaya R, El-Hajj Fuleihan Chiliad. Issue of antiepileptic drugs on bone density in ambulatory patients. Neurology. 2000 May 14;58(9):1348–53. [PubMed: 12011279]
-
Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. Washington (DC): American Society for Os and Mineral Research; 2003. cover.
-
Friedrichs WE, Reddy SV, Bruder JM, Cundy T, Cornish IJ, Vocaliser FR, Roodman GD. Sequence analysis of measles virus nucleocapsid transcripts in patients with Paget's affliction. J Bone Miner Res. 2002 Jan;17(1):145–51. [PubMed: 11771661]
-
Geurs NC, Lewis CE, Jeffcoat MK. Osteoporosis and periodontal disease progression. Periodontol 2000. 2003;32:105–10. [PubMed: 12756036]
-
Go T. Low-dose oral etidronate therapy for immobilization hypercalcaemia associated with Guillain-Barre syndrome. Acta Paediatr. 2001 Oct;90(10):1202–4. [PubMed: 11697437]
-
Haugeberg M, Orstavik RE, Kvien TK. Effects of rheumatoid arthritis on bone. Curr Opin Rheumatol. 2003 Jul;15(4):469–75. [PubMed: 12819477]
-
Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ. Alterations in bone characteristics associated with glycemic control in adolescents with blazon 1 diabetes mellitus. J Pediatr. 2004 Jan;144(1):56–62. [PubMed: 14722519]
-
Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its human relationship to oral os loss. Periodontol. 2000 Jun;2000:23, 94–102. [PubMed: 11276771]
-
Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ 3rd. Epidemiology and clinical features of osteoporosis in immature individuals. Bone. 1994 Sep-Oct;15(5):551–5. [PubMed: 7980966]
-
Khosla South, Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A cardinal office for bioavailable estrogen. J Clin Endocrinol Metab. 1998 Jul;83(7):2266–74. [PubMed: 9661593]
-
Khosla South, Melton J third. Fracture take a chance in main hyperparathyroidism. J Os Miner Res. 2002 November;17(Suppl two):N103–7. [PubMed: 12412786]
-
Kiratli BJ. Immobilization osteopenia. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. Second Edition. Vol. 2. San Diego (CA): Academic Press; 2001. pp. 207–27.
-
Lien Grand, Flato B, Haugen M, Vinje O, Sorskaar D, Dale K, Johnston V, Egeland T, Forre O. Frequency of osteopenia in adolescents with early-onset juvenile idiopathic arthritis: A long-term outcome written report of i hundred five patients. Arthritis Rheum. 2003 Aug;48(viii):2214–23. [PubMed: 12905475]
-
Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa Yard, Langman C, Malluche HH, McCarthy JT, Massry SG, Mehls O, et al. Diagnosis, cess, and treatment of bone turnover abnormalities in renal osteo-dystrophy. Am J Kidney Dis. 2004 Mar;43(3):558–65. [PubMed: 14981615]
-
Melton LJ tertiary, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA. Fracture take chances in monoclonal gammopathy of undetermined significance. J Bone Miner Res. 2004 Jan;19(1):25–30. [PubMed: 14753733]
-
Michaelsson Chiliad, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003 Jan 23;348(four):287–94. [PubMed: 12540641]
-
Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P. Bone mineral density in women with depression. N Engl J Med. 1996 October 17;335(16):1176–81. [PubMed: 8815939]
-
Morales-Piga AA, Rey-Rey JS, Corres-Gonzalez J, Garcia-Sagredo IM, Lopez-Abente G. Frequency and characteristics of familial aggregation of Paget's affliction of bone. J Bone Miner Res. 1995 Apr;ten(iv):663–seventy. [PubMed: 7610939]
-
National Osteoporosis Foundation. Osteoporosis: What is it? [homepage on the Internet]. Washington, DC: National Osteoporosis Foundation; [Cited 2004 Mar ane]. Available from: http://www
.nof.org/osteoporosis/index .htm. -
Netter Frank H. The Ciba drove of medical illustrations. In: Woodburne Russell T, Crelin Edmund Southward, Kaplan Frederick S., editors. Musculoskeletal system: Anatomy, Physiology, and Metabolic Disorders. Function 1. Vol. 8. West Cauldwell, NJ: Ciba-Geigy Pharmaceutical Products; 1987. p. 260.
-
Norman ME. Juvenile osteoporosis. In: Favus, MJ, editor. Primer on the metabolic os diseases and disorders of mineral metabolism. 5. Washington, DC: American Society for Bone and Mineral Research; ; 2003. pp. 382–half-dozen.
-
Orstavik RE, Haugeberg 1000, Uhlig T, Mowinckel P, Falch JA, Halse JI, Kvien TK. Self reported non-vertebral fractures in rheumatoid arthritis and population based controls: Incidence and human relationship with bone mineral density and clinical variables. Ann Rheum Dis. 2004 Feb;63(2):177–82. [PMC free article: PMC1754879] [PubMed: 14722207]
-
Orwoll E. Osteoporosis in men. Endocrinol Metab Clin North Am. 1998 Jun;27(2):349–67. [PubMed: 9669142]
-
Ott S, Aitken ML. Osteoporosis in patients with cystic fibrosis. Clin Chest Med. 1998 Sep;19(3):555–67. [PubMed: 9759556]
-
Pavelka Chiliad. Osteonecrosis. Baillieres Best Pract Res Clin Rheumatol. 2000 Jun;14(2):399–414. [PubMed: 10925752]
-
Pettifor JM. Rickets. Calcif Tissue Int. 2002 May;70(5):398–9. [PubMed: 11960205]
-
Pettifor JM. Nutritional and drug-induced rickets and osteomalacia. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. Washington, DC: American Order for Os and Mineral Inquiry; 2003. pp. 399–407.
-
Piepkorn B, Kann P, Forst T, Andreas J, Pfützner A, Beyer J. Bone mineral density and bone metabolism in diabetes mellitus. Horm Metab Res. 1997 Nov;29(11):584–91. [PubMed: 9479561]
-
Poole KE, Reeve J, Warburton EA. Falls, fractures, and osteoporosis after stroke: Time to think about protection. Stroke. 2002 May;33(5):1432–six. [PubMed: 11988628]
-
Ramsey-Goldman R, Dunn JE, Huang CF, Dunlop D, Rairie JE, Fitzgerald Southward, Manzi South. Frequency of fractures in women with systemic lupus erythematosus: Comparing with U.s.a. population data. Arthritis Rheum. 1999 May;42(five):882–xc. [PubMed: 10323443]
-
Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004 Apr 24;363(9418):1377–85. [PubMed: 15110498]
-
Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to os loss in crumbling men. J Os Miner Res. 1998 May;xiii(5):763–73. [PubMed: 9610739]
-
Riggs BL, Khosla S, Melton LJ tertiary. Sex activity steroids and the construction and conservation of the developed skeleton. Endocr Rev. 2002 Jun;23(iii):279–302. [PubMed: 12050121]
-
Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001 Jun;49(half-dozen):732–6. [PubMed: 11454111]
-
Roodman GD. Biology of osteoclast activation in cancer. J Clin Oncol. 2001 Aug 1;19(15):3562–71. [PubMed: 11481364]
-
Ross DS. Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid. 1994 Autumn;4(3):319–26. [PubMed: 7833670]
-
Saag K. Glucocorticoid-induced osteoporosis. Endocrinol Metab Clin North Am. 2003 Mar;32(one):135–57. vii. [PubMed: 12699296]
-
Sato Y, Asoh T, Kaji K, Oizumi K. Beneficial result of intermittent cyclical etidronate therapy in hemiplegic patients following an acute stroke. J Bone Miner Res. 2000 Dec;15(12):2487–94. [PubMed: 11127214]
-
Schneider A, Shane Eastward. Osteoporosis secondary to affliction and medications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd ed. San Diego: Bookish Press; 2001. pp. 303–27.
-
Seeman E. Invited Review: Pathogenesis of osteoporosis. J Appl Physiol. 2003 Nov;95(5):2142–51. [PubMed: 14555675]
-
Sheth RD. Bone health in epilepsy. Epilepsia. 2002 Dec;43(12):1453–4. [PubMed: 12460244]
-
Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, et al. Skeletal disease in principal hyperparathyroidism. J Bone Miner Res. 1989 Jun;4(three):283–91. [PubMed: 2763869]
-
Silverberg SJ, Bilezikian JP. Clinical presentation of principal hyperparathyroidism in the Us. In: Bilezikian JP, Marcus R, Levine MA, editors. The parathyroids. 2d ed. San Diego (CA): Academic Printing; 2001. pp. 349–60.
-
Siris ES, Ottman R, Flaster E, Kelsey JL. Familial aggregation of Paget'due south affliction of bone. J Os Miner Res. 1991 May;6(v):495–500. [PubMed: 2068956]
-
Siris ES, Roodman GD. Paget'southward disease of bone. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. v. Washington, DC: American Order for Bone and Mineral Enquiry; 2003. pp. 495–506.
-
Smith MR. Diagnosis and direction of treatment-related osteoporosis in men with prostate carcinoma. Cancer. 2003 Feb 1;97(3 Suppl):789–95. [PubMed: 12548577]
-
Stein E, Shane E. Secondary osteoporosis. Endocrinol Metab Clin Due north Am. 2003 Mar;32(i):115–34. 7. [PubMed: 12699295]
-
Tannenbaum C, Clark J, Schwartzman One thousand, Wallenstein S, Lapinski R, Meier D, Luckey Yard. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002 Oct;87(10):4431–7. [PubMed: 12364413]
-
Thomas J, Doherty SM. HIV infection—A take a chance factor for osteoporosis. J Acquir Allowed Defic Syndr. 2003 Jul i;33(3):281–91. [PubMed: 12843738]
-
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr. The role of the Wnt-signaling adversary DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003 Dec 25;349(26):2483–94. [PubMed: 14695408]
-
Tuzun S, Altintas A, Karacan I, Tangurek S, Saip South, Siva A. Bone status in multiple sclerosis: Beyond corticosteroids. Mult Scler. 2003 Dec;nine(vi):600–four. [PubMed: 14664473]
-
van Staa TP, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002 Oct;13(ten):777–87. [PubMed: 12378366]
-
Wermers RA, Khosla Due south, Atkinson EJ, Hodgson SF, O'Fallon WM, Melton LJ tertiary. The rise and fall of main hyperparathyroidism: A population-based study in Rochester, Minnesota, 1965–1992. Ann Intern Med. 1997 Mar 15;126(6):433–40. [PubMed: 9072928]
-
WHO Scientific Group on the Burden of Musculoskeletal Weather at the Offset of the New Millennium. The brunt of musculoskeletal conditions at the start of the new millennium: Study of a scientific group. Vol. 919. Geneva, Switzerland: World Health Organisation; 2003. p. 57. technical report series. [PubMed: 14679827]
-
Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS. Low, falls, and risk of fracture in older women. Written report of Osteoporotic Fractures Research Group. Arch Intern Med. 1999 Mar 8;159(5):484–ninety. [PubMed: 10074957]
-
Whyte MP. Sclerosing os disorders. In: Favus, MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. Washington, DC: American Society for Os and Mineral Research ; 2003. pp. 449–66.
-
Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: Clinical and pathological features in 43 patients. Medicine. 1992 Jul;71(4):197–205. [PubMed: 1518393]
Source: https://www.ncbi.nlm.nih.gov/books/NBK45506/#:~:text=The%20most%20common%20form%20of,bone%20structure%20as%20people%20age.
Posted by: reyesyesper.blogspot.com


0 Response to "Which Condition Is Characterized By Extensive Bone Destruction Followed By Abnormal Bone Repair"
Post a Comment