How To Remove Jelly Bumps From Contact Lenses
The How and Why of Contact Lens Deposits
Optometrists need a comprehensive agreement of this complication to help patients avoid it.
By Heidi Wagner, OD, MPH

Release Date: May 15, 2022
Expiration Appointment: May 15, 2023
Estimated time to complete activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group.
Educational Objectives: Afterwards completing this activeness, the participant should exist better able to:
- Discuss the underlying mechanisms of contact lens deposits.
- Identify contact lens deposits in their patients.
- Recommend changes to reduce deposits in their contact lens wearers.
- Factor in lens textile choices to improve condolement and vision.
- Draw how lens care options and surface treatments affect deposition.
Target Audience: This activity is intended for optometrists engaged in the care of patients with contact lens deposits.
Accreditation Argument: In back up of improving patient care, this activity has been planned and implemented by the Postgraduate Found for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Instruction, the Accreditation Quango for Chemist's shop Education, and the American Nurses Credentialing Eye, to provide continuing educational activity for the healthcare team. Postgraduate Found for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Heidi Wagner, OD, MPH, Ohio State University
Credit Argument: This course is COPE approved for 2 hours of CE credit. Class ID is 67923-CL. Check with your local land licensing board to come across if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Wagner has contracted research with Alcon and has received honoraria from Wink Productions.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Teaching Group planners, managers and editorial staff have nothing to disclose.
In this era of disposability, many centre intendance providers are less concerned about contact lens deposits. In 2022, daily disposable soft contact lenses (SCLs) accounted for 35% of international lens prescribing and 44% of lenses prescribed in the Us.1 As the market place share of conventional and planned replacement SCLs shrinks, lens deposits may exist less prevalent and less astringent; still, lens deposition remains a gene, particularly with the expanded use of specialty contact lenses.
Specialty SCLs, gas permeable (GPs) lenses and hybrids play an important part in the United states of america market of 45 million contact lens wearers.2 Specialty SCLs and hybrids are typically replaced far less frequently than daily disposables—frequently at quarterly or six-calendar month intervals. In contrast to SCLs, GPs are oftentimes replaced "reactively" (i.e., when the patient requires a change in lens ability or experiences reduced condolement, degraded vision or lens loss or damage) rather than on a planned schedule.
Contact lens deposits significantly impact the patient'south lens wearing feel and ocular health. Lens spoilage can potentially reduce lens surface wettability and adversely impact patient comfort, wearing time and quality of vision. Further, lens deposits can result in contact lens-related ocular pathology, including papillary conjunctivitis, punctate keratitis, corneal inflammatory events and even microbial keratitis.3,four This article reviews how to identify various types of lens deposits, describes the touch on of lens fabric choices on comfort and vision and delineates how lens care options and surface treatments affect degradation.
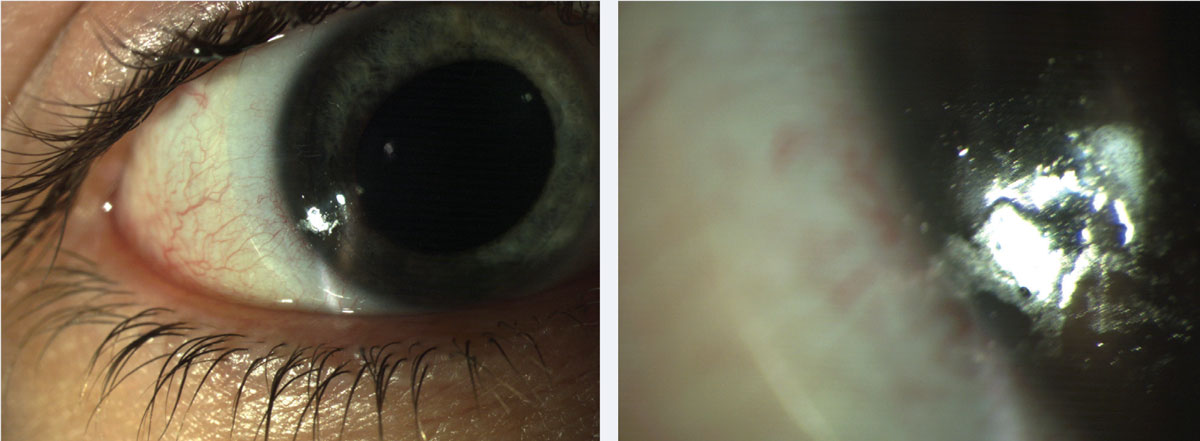 |
| Fig. 1. Patients can present with both protein and lipid deposits. These common lens deposits are shown hither at (left) low and (right) high magnification. Click epitome to enlarge. |
Underlying Mechanisms
A full general agreement of the underlying mechanisms of lens deposits and an awareness of strategies to reduce them remain integral to contemporary contact lens do. Lens depositing is influenced by many factors, including patient compliance, individual tear chemistry and environment. Individual tear chemistry is evidenced by lipid composition, protein profile, mucin and electrolyte analysis—characteristics that manifest in the wearer response. 5 Agreement these interactions can help the center intendance provider optimize lens performance and minimize adverse events.
Identifying Deposits
Lens deposition begins inside minutes of wear. 6 While surface deposits may be minimized past increasing the frequency of lens replacement, variation exists among individual patients with regards to tear chemistry and compliance with the lens intendance regimen. seven Practitioners must be vigilant in identifying lens deposits with all types of lens materials and replacement regimens.
Contact lens deposits are best identified through observation of the lens on the centre with biomicroscopy under varying illumination and magnification. A lens loupe is a practical alternative, especially if the lens is damaged and could potentially impairment the centre.
Lens deposits tin can be distinguished by color, structure and location. Identification of the predominant eolith can guide the practitioner in direction decisions. There are a number of common types of deposits practitioners should be enlightened of.
Proteins and lipids. These are long-recognized lens deposits in contact lens exercise.8,9 Poly peptide deposits occur every bit lysozyme binds to the lens surface and undergoes structural changes that impair its part. These changes, termed protein denaturation, are influenced by numerous factors such as the lens substrate, pH and temperature.six Protein deposits are characterized by an opaque film on the lens that becomes more than obvious over fourth dimension. In contrast, lipid deposition is characterized past a shiny, lubricious appearance. Both are present in SCLs and GPs. Protein and lipid deposits can be observed in combination in an individual patient (Figure 1).
Lens calculi. Sometimes referred to as "jelly bumps," these deposits are distinct, localized elevations on the anterior surface of the SCL. Lens calculi are equanimous of calcium, lipid and mucoprotein inherent in the tear film (Figure 2).10 Their formation is attributed to depletion of the aqueous tear layer that results in a hydrophobic zone that, in turn, promotes deposition.xi If pregnant in number and size, they can degrade condolement and vision.
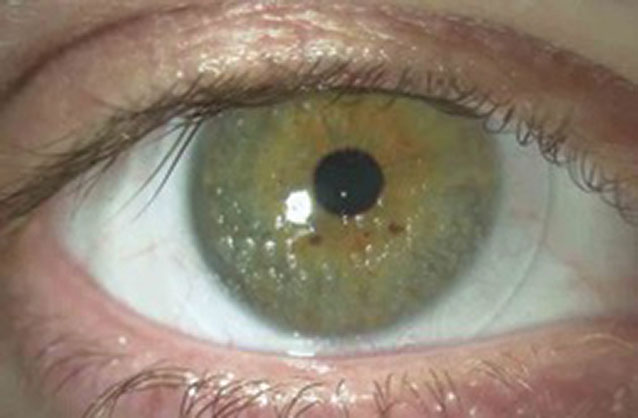 |
| Fig. ii. This patient presented with distinct, localized elevations on the anterior surface of the lens—lens calculi. Click paradigm to enlarge. |
While frequently observed in the era of conventional SCL lens clothing, they are relatively uncommon in lenses that are replaced monthly or more ofttimes. Thus, if practitioners observe lens calculi in patients wearing lenses with shorter replacement cycles, wearers may exist "stretching" their replacement cycles. As the deposit is embedded within the matrix of the lens, replacement is necessary.
This blazon of deposit is more commonly observed in loftier-h2o, ionic (group 4), hydrogel lens materials.eleven The practitioner can accost this problem by refitting the patient into a different lens material, though simply reinforcing the lens replacement schedule or refitting into daily disposable contact lenses may accost the problem.
Fungal deposits. Given the predilection of mucus for lens materials of a higher water content, this type of deposit, which is often characterized by a filamentary appearance, is more commonly observed in soft or hybrid lenses.12
Fungal deposits may exist associated with poor disinfection regimens. This can occur when patients use saline instead of a multipurpose disinfection solution or when function-time wearers or multiple pair (e.g., colored lenses) wearers store lenses in solution for extended periods of time. Additionally, patients who disinfect lenses with hydrogen peroxide systems may be unaware that the neutralized disinfection solution is saline and that the solution must be replaced every 7 days if the lenses are not worn.13 Therefore, it is important to prescribe a lens care system that is advisable for the patient's wearing schedule and ensure that the patient understands how to employ it.
Iron deposits. Characteristically circular and brown-to-orange in color, such deposition may be a consequence of incorporating tap water into the lens care regimen, despite published evidence of the association of Acanthamoeba with water exposure.14 In a survey of more than than 1,000 SCL wearers, 31% reported rinsing their SCLs with tap water on at to the lowest degree one occasion, and x% reported e'er or fairly often rinsing their lenses with tap h2o.xv Of the wearers who reported rinsing their lenses with tap water, 41% reported besides storing their lenses in tap water.15
Upon identifying iron deposits, eye care providers should emphasize that no corporeality of water exposure is acceptable. This message may be reinforced by promotional materials, such as the "no water" stickers distributed by the Cornea, Contact Lenses and Refractive Technologies Diplomate Section of the American Academy of Optometry (Figure three).
 |
| Fig. three. These "no h2o" stickers, distributed by the American Academy of Optometry, can help reinforce to patients that no amount of water exposure is acceptable. Click image to enlarge. |
Mucin balls. These deposits are round, semitransparent spheres ranging in size between 40µm and 120µm. While mucin balls have been observed in a variety of lens materials, they are more oft associated with silicone hydrogels (SiHy). Inquiry suggests that their formation is based on a mechanical interaction between the cornea and high modulus SiHy materials.16
Mucin balls practice not appear to impact vison or comfort and, therefore, can hands be differentiated from other types of lens deposits. They are more than likely observed in first-generation SiHy products that are characterized by "stiffer" (high modulus) lens materials.
Environmental debris. Make-upwardly, such every bit mascara and eyeliner, is a mutual source of lens deposits. While eye make-up may be hands identified by colour and texture, identifying the source of other contaminants degrading the lens surface may show to be more elusive.17
Lotions transferred from fingertips and aerosol hairspray can also demark to the lens. These types of deposits can exist eliminated by proper hand washing earlier lens treatment and applying make-up subsequently lens insertion (Effigy 4).
Other potential sources of environmental lens deposits include organic debris such as leafage litter and inorganic contaminants such as a metal foreign body. If you doubtable a metallic strange object, ever perform a more extensive middle exam, given the possibility of an intraocular strange torso.
Lipid, protein and exogenous contaminants are likely to eolith on both GP and SCL lens materials. Unique to GP lenses, however, is poor wetting exhibited in newly dispensed lenses. This is somewhat less mutual as water-soluble products currently used in the manufacturing process accept largely eliminated the oily residuum (i.eastward., "pitch") that was previously part of the manufacturing process.
This problem can generally be solved by plasma cleaning or soaking lenses in an appropriate conditioning solution prior to dispensing. Lens cleaners can also be used with advisable materials, as discussed beneath.
On occasion, topical and systemic medications have been associated with lens discoloration in SCLs. For instance, rifampin, a drug used to care for tuberculosis, tin can cause an orange discoloration of contact lenses.18 A similar phenomenon has been reported with sulfasalazine, which is used to manage inflammatory bowel illness.19 Lens discoloration, ranging from pink to brown, has also been observed with some topical medications, such as the epinephrine ophthalmic drops used in the by to care for glaucoma.
While these conditions are not observed in every 24-hour interval clinical practice, the practitioner should exist aware of the potential of oral and topical medications to influence the tear ocular environs.20
Lysozyme deposits. Notably, lysozyme deposition may provide benign furnishings during contact lens wear, as lysozyme exhibits antibacterial and anti-inflammatory backdrop.6 Research too shows that lactoferrin in the tears has the potential to work in concert with lysozyme to inhibit gram-positive and gram-negative leaner.6 However, farther study is needed to ameliorate understand these interactions.
Lens materials influence the deposition of tear-derived products that, in turn, influences lens comfort.21 Some investigators have also challenged the belief that lens deposition negatively impacts condolement, noting that lysozyme has, on occasion, been associated with increased condolement in HEMA-based lens materials. This was attributed to the fact that lysozyme retains a higher degree of activity when deposited on traditional hydrogel lens materials compared with silicone hydrogels.22 They propose the development of lens materials that tin can selectively bind "good" deposits and inhibit "bad" deposits.23
Lens Material
The FDA classifies hydrogel contact lenses as ionic (groups III and IV) and nonionic (groups I and II). Groups II and IV showroom a higher h2o content (≥fifty% h2o) than groups I and III. SiHy, in full general, are characterized by lower h2o content only higher oxygen permeability. half dozen
The rate of protein deposition is significantly related to the lens material. Polymethyl methacrylate (PMMA) and SiHy lens materials eolith less lysozyme than hydrogels, and lysozyme is particularly prevalent in high-water, ionic lens (group Four) materials.24 The external environs and lens handling farther expose the lenses to contaminants.
SCLs provide an platonic medium to attract lens deposits, given the hydrophilic surface. Hydrogel lenses incorporate methacrylic acrid to increment h2o content and oxygen permeability.25 Consequently, HEMA-based lens materials exhibit a predisposition toward protein degradation, as the negatively charged methacrylic acid binds to positively charged proteins, including lysozyme.26 Thus, refitting patients wearing SCLs from high-water, ionic lens (group IV) materials to low-water, non-ionic lens (group I) materials may reduce poly peptide deposits. SiHy lenses, while highly oxygen permeable, are potentially hydrophobic in nature. They may exhibit reduced wettability and a greater tendency towards lipid deposition compared with their HEMA-based counterparts.27
Rigid lens materials exhibit a parallel story. All just obsolete, PMMA contact lenses were deposit resistant but impervious to oxygen. GP lenses are permeable to oxygen in varying degrees based on the polymer components. Silicone was added to the lens material to create silicone acrylate (SA) lens materials. This resulted in an increased oxygen permeability just more protein deposition.
Fluorine was so added to maintain oxygen permeability and improve wettability of the current generation of fluorosilicone acrylate (FSA) lens materials. Before generation SA lenses tended to deposit proteins while newer FSA lenses tend to eolith lipids.28
Given these various textile characteristics, clinicians should customize the lens material to the individual patient. For example, a hyper-Dk lens cloth may be desirable for overnight clothing in orthokeratology while a moderate-Dk lens textile may exist ideal for a patient who tends to deposit lipids.
In improver, the provider tin can further tailor the lens care regimen to the needs of the lens wearer. For example, a heavy lipid depositor who too requires a high-Dk lens cloth could benefit from a more rigorous lens intendance organization every bit described below.
Further consideration for giant papillary conjunctivitis (GPC) is warranted, given its association with lens deposits. GPC is most commonly associated with SCLs but tin can be associated with GPs, as well every bit sutures following surgery.three While the condition was initially described as a "reaction" to soft contact lenses, the term was afterward redefined by researchers who postulated that the syndrome was immunologic in origin with deposits on the lenses serving as an antigen (type IV immune response).35 A mechanical component has besides been suggested, although there is no consensus on this upshot.36
GPC is characterized by papillae on the tarsal conjunctiva. In balmy cases, patients may have symptoms of lens awareness. In severe cases of GPC, patients may experience excessive lens movement, substantial lens depositing and lens intolerance. Contact lens-induced GPC can be managed by increasing lens replacement frequency, decreasing lens wearing time or changing lens materials. Concurrent pharmacological management, such every bit mast-cell inhibitors/antihistamine combination drugs and topical steroids, can be added.
Lens Wear and Care
Proper contact lens wear and intendance practices are essential for all contact lens modalities, and they should be tailored to the particular lens modality and patient. In a recent survey administered past the Centers for Affliction Control and Prevention, six of seven contact lens wearers acknowledged at least one beliefs that places them at risk for a contact lens-related adverse event. 2 Eye intendance providers play an important part in educating all contact lens wearers at the initial plumbing equipment also as reinforcing all-time practices at every follow-up visit.
Clinicians should provide specific guidance based on the unique needs of the patient, including the lens material, replacement schedule, contact lens care, tear chemistry and history of compliance.
SCLs. Advisable lens care goes a long way in maintaining a clean lens surface. Chemical disinfection systems (normally designated every bit multipurpose solution [MPS]) combine cleaning, rinsing and disinfection. While MPS is integral to lens care, it is useful to remember that its success is based on its power to evangelize key components of the lens care regimen: cleaning, rinsing, disinfecting and storage.
Cleaning removes loosely adhered deposits, as does lubrication. Rinsing removes the droppings and avoids the introduction of additional external contaminants. Proper disinfection and storage limits microbial intrusion. It is important that patients recollect that all lens care components—including the lens example, when applicable—are part of the lens intendance system.
In studies where the FDA required the manufacturers to inoculate the lenses with one million organisms to study the efficacy of a lens care arrangement, the inclusion of a cleaning step removed 1 log unit of microorganisms from the lens. If the cleaner was rinsed from the lens, ii additional log units of microorganisms were further eliminated.17
This work reinforces the need for digital cleaning, even with MPS. The FDA further discouraged the promotion of "no rub" lens intendance systems afterwards the voluntary removal of two lens care products from the marketplace following their association with Fusarium and Acanthamoeba.29-31 Further studies accept supported digital rubbing and rinsing to minimize deposits and limit bacterial contagion in reusable soft and GP lenses.32,33
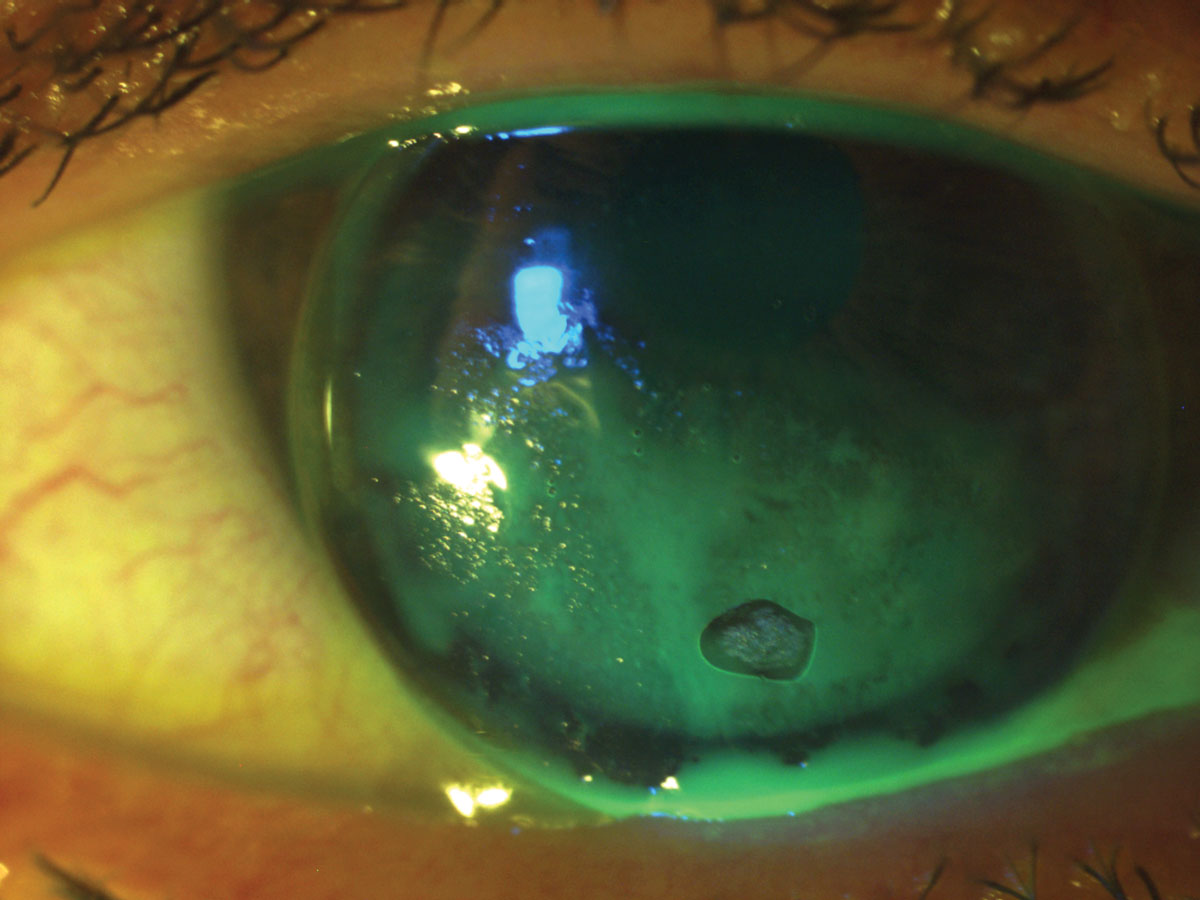 |
| Fig. 4. In this case, lip lotion was inadvertently transferred to the lens, resulting in poor GP lens wetting. Click image to overstate. |
A separate surfactant or enzymatic cleaner is rarely indicated for two-calendar week or monthly replacement SCLs, although these products may be added to the intendance regimen for "heavy depositors." Surfactant cleaners remove loosely adhered lens debris, unbound poly peptide and microbial contamination. Every bit these cleaners are less attainable than in the by, patients may crave additional direction regarding where to buy them.
Hydrogen peroxide systems are a particularly effective preservative-free disinfection selection. Contemporary systems contain a surfactant and, in one system, a wetting agent. However, anecdotal reports suggest lens remainder may exist associated with solutions that contain a proprietary wetting amanuensis. This can exist resolved by switching to another hydrogen peroxide product without the wetting agent.
Practitioners should exist cognizant of current MPS systems, make an initial prescribing decision and modify as needed. They should besides be alerted to potential patient pitfalls, such as "topping off" (which tin can reduce disinfection efficacy), purchasing alternate products and non completing the cleaning regimen equally directed. It should also be noted that SCL wearers who take an aplenty supply of lenses are more than likely to replace their lenses at recommended intervals.34
GPs. I-bottle care systems for cleaning, rinsing, disinfection and storage are also available for GP lenses. As with MPS SCL solutions, digital cleaning can enhance the efficacy of the process. For example, Unique pH (Menicon) and Boston Simplus (Bausch + Lomb) provide one-bottle convenience. Two-canteen systems, such as Boston Original and Accelerate (Bausch + Lomb), contain a split abrasive cleaner that enhances the cleaning regimen. Boston Original was designed for lower-Dk SA lenses, which tend to deposit proteins, while Boston Advance was adult for higher-Dk FSA lens materials that deposit lipids.
Clinicians can also manage lens deposits past being judicious in the addition of Hydra-PEG (Tangible Science) and surface treatments. Hydra-PEG is a biocompatible polymer that may be applied to GP or hybrid lenses as function of the manufacturing procedure. Every bit described by the manufacturer, the coating promotes a lubricious lens surface that is designed to inhibit lens deposits and fogging. Tangible Clean (Tangible Science) is an MPS solution designed for Hydra-PEG coated lenses. It tin can besides be used for uncoated lenses.
Abrasive cleaners are contraindicated in plasma-treated lenses, hyper-Dk lens materials and with Hydra-PEG. Non-abrasive cleaners that comprise booze are peculiarly effective with lipid removal and are compatible with hyper-Dk lens materials; however, no consensus exists regarding their use with plasma-treated lenses, and they are contraindicated with Hydra-PEG.
Given that tap h2o is contraindicated with all contact lenses, low viscosity solutions such every bit saline or MPS should be employed to rinse the cleaner from the lens. Every bit this inadvertently introduces a third stride, one-pace hydrogen peroxide systems provide a practical alternative whereby the disinfection solution likewise contains a surfactant cleaner and the solution neutralizes to saline.
Anecdotally, patients who successively apply MPS systems with corneal GPs may crave a more than rigorous organization with scleral lenses, presumably because corneal lenses exhibit more tear exchange. Heavy depositors may too benefit from periodic cleaners such equally enzymatic cleaners that remove protein or Progent (Menicon), which exhibits both cleaning and disinfection properties. Progent may be used as frequently as every two weeks for heavy depositors and can exist used in office. Patients who feel difficulty digitally cleaning their lenses because of the lens geometry (e.1000., lenses for keratoconus with steep base curves) may also benefit from incorporating periodic cleaners.
Contact lens deposits are a well-known clinical challenge. This challenge can lead to reduced comfort and vision and negatively impact ocular health. Often, irresolute the contact lens or care regimen is not enough to ward off deposits. Many factors impact a patient's chances of experiencing this complication, including the lens cloth, surface treatments, wear schedules, care regimens and the patient's individual tear fluid limerick.
Source: https://www.reviewofcontactlenses.com/article/the-how-and-why-of-contact-lens-deposits

0 Response to "How To Remove Jelly Bumps From Contact Lenses"
Post a Comment